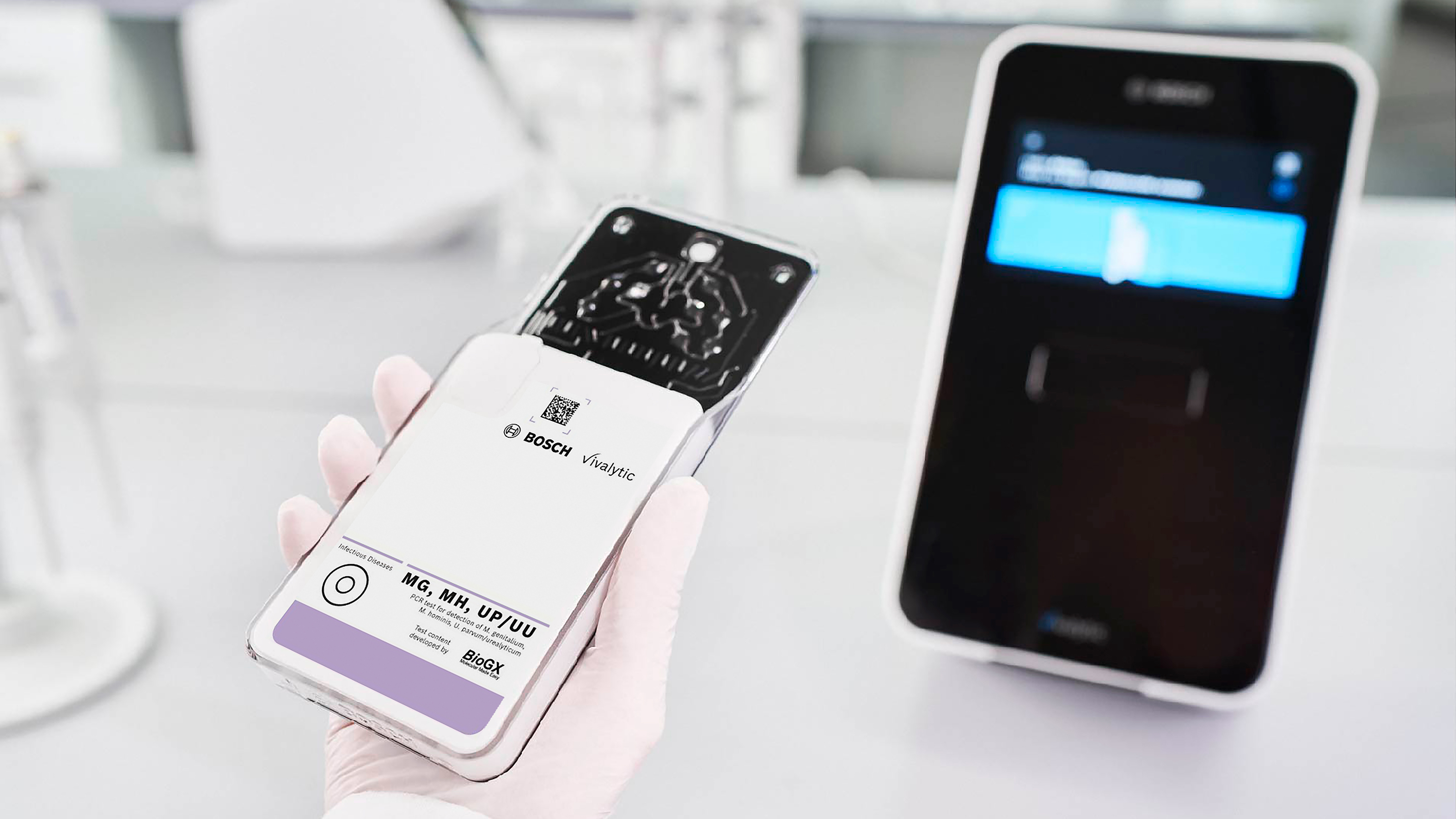
Waiblingen – Bosch Healthcare Solutions has received CE-label for the new Vivalytic MG, MH, UP/UU, developed in partnership with BioGX, a US-based global molecular diagnostics company. The test is now available as part of the Vivalytic analyser test portfolio and can thus be used directly at the point of care. This real-time PCR rapid test can detect four pathogens of sexually transmitted infections (STIs): Mycoplasma genitalium (MG), Mycoplasma hominis (MH), Ureaplasma parvum (UP), and Ureaplasma urealyticum (UU). After taking the sample and starting the analysis, a positive result is available in as little as an hour, making the test particularly suitable for use in direct patient care at doctors’ offices and clinics. Here, it can enable STI differential diagnosis directly at the point of sampling without delays due to transportation or logistics. For example, the test can detect in case of infectious urethritis (non-gonorrheal urethritis, NGU)1, if one of the four pathogens is responsible for the infection. However, rapid diagnosis also helps to counteract the further spread of STI pathogens. The Vivalytic MG, MH, UP/UU test saves a lot of time compared to diagnostics by cultivation of the pathogenic germs. In the case of MH, UP, and UU, cultivation can take one to three days, and up to six months for MG2.
Sources
1) G. Bonkat et al., EAU guidelines on urological infections; European Association of Urology 2020
2) CDC (Centers for Disease Control and Prevention) Guidelines 2021, Publication: Workowski et al., Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021 Jul 23;70(4):1-187
3) https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis), at: 15.03.2022
4) Waites et al., J Mol Diagn. 2012 Sep;14(5):437-50
YouTube
Bosch Healthcare Solutions GmbH is a wholly owned subsidiary of Robert Bosch GmbH. The subsidiary was established in 2015 with the aim of developing products and services that improve people’s health and quality of life. Nearly 300 associates (state 2024) are employed at the company’s headquarters in Waiblingen, Germany. The subsidiary’s solutions draw on the Bosch Group’s core competencies: sensors to collect data, software to evaluate that data, and services based on this data analysis.
Additional information is available online at www.bosch-healthcare.com, www.vivatmo.com, www.bosch-vivalytic.com.
The Bosch Group is a leading global supplier of technology and services. It employs roughly 412,000 associates worldwide (as of December 31, 2025). According to preliminary figures, the company generated sales of 91 billion euros in 2025. Its operations are divided into four business sectors: Mobility, Industrial Technology, Consumer Goods, and Energy and Building Technology. With its business activities, the company aims to use technology to help shape universal trends such as automation, electrification, digitalization, connectivity, and an orientation to sustainability. In this context, Bosch’s broad diversification across regions and industries strengthens its innovativeness and robustness. Bosch uses its proven expertise in sensor technology, software, and services to offer customers cross-domain solutions from a single source. It also applies its expertise in connectivity and artificial intelligence in order to develop and manufacture user-friendly, sustainable products. With technology that is “Invented for life,” Bosch wants to help improve quality of life and conserve natural resources. The Bosch Group comprises Robert Bosch GmbH and its roughly 490 subsidiary and regional companies in over 60 countries. Including sales and service partners, Bosch’s global manufacturing, engineering, and sales network covers nearly every country in the world. Bosch’s innovative strength is key to the company’s further development. At 136 locations across the globe, Bosch employs some 82,000 associates in research and development.
Additional information is available online at www.bosch.com, www.bosch-press.com.